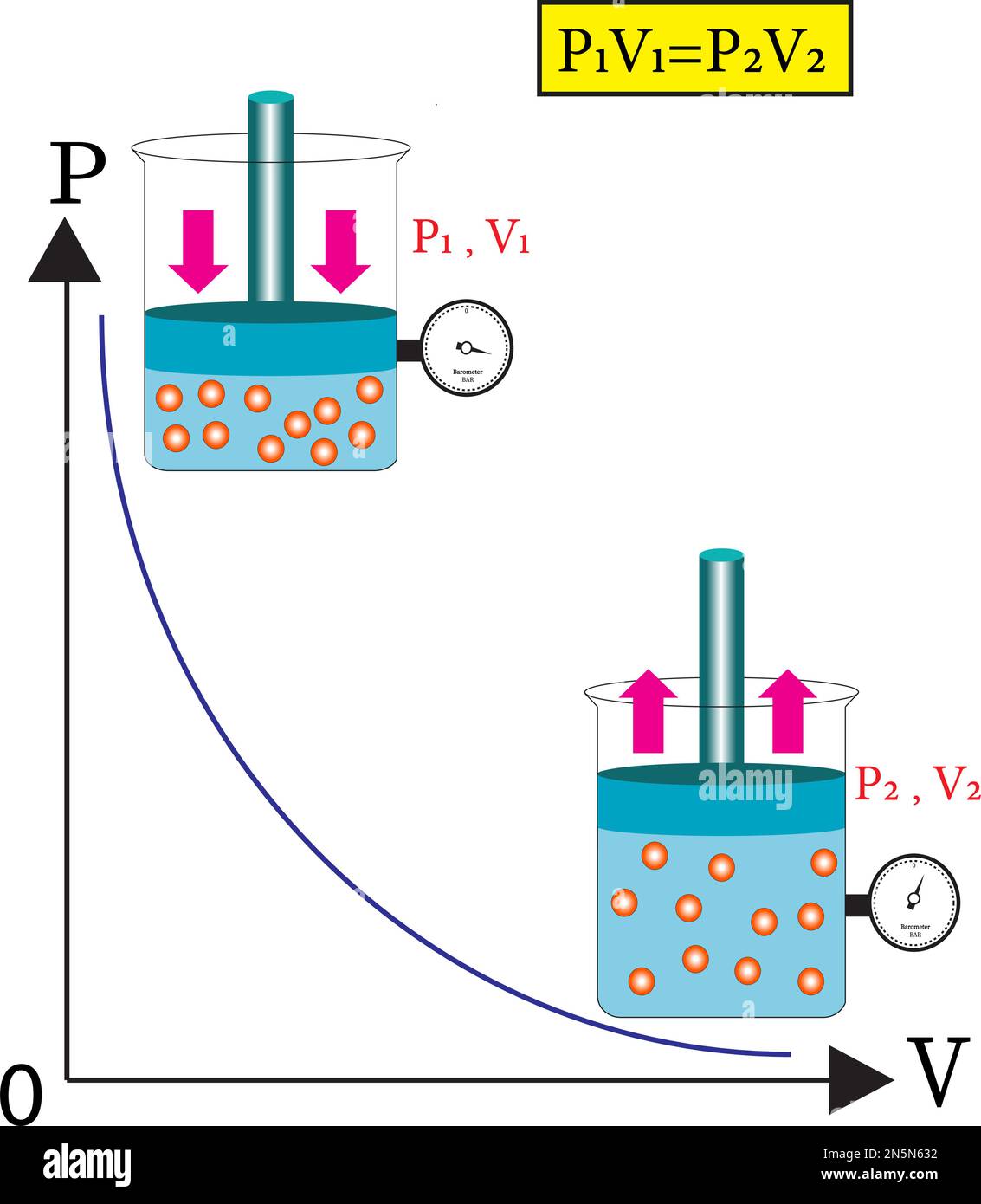
What Is The Relationship Between Gas Pressure And Volume . When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. define the relationship between gas volume and pressure, boyle's law. When it occupies a larger volume, it exerts a lower pressure. when a gas occupies a smaller volume, it exerts a higher pressure; the relationship between pressure and volume: learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize. when a gas occupies a smaller volume, it exerts a higher pressure; As the pressure on a gas increases, the volume of the gas. Use boyle's law to calculate. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number.
from www.alamy.com
When it occupies a larger volume, it exerts a lower pressure. define the relationship between gas volume and pressure, boyle's law. When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. when a gas occupies a smaller volume, it exerts a higher pressure; the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. the relationship between pressure and volume: As the pressure on a gas increases, the volume of the gas. when a gas occupies a smaller volume, it exerts a higher pressure; Use boyle's law to calculate. learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize.
Boyle's Law , Relationship between pressure and gas volume Stock Vector
What Is The Relationship Between Gas Pressure And Volume when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. when a gas occupies a smaller volume, it exerts a higher pressure; the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. define the relationship between gas volume and pressure, boyle's law. When it occupies a larger volume, it exerts a lower pressure. the relationship between pressure and volume: Use boyle's law to calculate. As the pressure on a gas increases, the volume of the gas. learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize. when a gas occupies a smaller volume, it exerts a higher pressure;
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial What Is The Relationship Between Gas Pressure And Volume define the relationship between gas volume and pressure, boyle's law. learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. Use boyle's law to calculate. when a. What Is The Relationship Between Gas Pressure And Volume.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint What Is The Relationship Between Gas Pressure And Volume learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. As the pressure on a gas increases, the volume of the gas. when a gas occupies a smaller. What Is The Relationship Between Gas Pressure And Volume.
From chart-studio.plotly.com
Boyle's Law PressureVolume Relationship in Gases scatter chart made What Is The Relationship Between Gas Pressure And Volume define the relationship between gas volume and pressure, boyle's law. when a gas occupies a smaller volume, it exerts a higher pressure; the relationship between pressure and volume: the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. learn about and revise particle motion,. What Is The Relationship Between Gas Pressure And Volume.
From saylordotorg.github.io
Relationships among Pressure, Temperature, Volume, and Amount What Is The Relationship Between Gas Pressure And Volume Use boyle's law to calculate. When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. define the relationship between gas volume and pressure, boyle's law. the relationship between pressure and volume: When it occupies a larger volume, it exerts a lower pressure. learn about and revise particle motion, gas pressure and the. What Is The Relationship Between Gas Pressure And Volume.
From masterconceptsinchemistry.com
What’s the relationship between pressure and volume of gas? Core What Is The Relationship Between Gas Pressure And Volume the relationship between pressure and volume: when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. Use boyle's law. What Is The Relationship Between Gas Pressure And Volume.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing What Is The Relationship Between Gas Pressure And Volume when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. As the pressure on a gas increases, the volume of the gas. define. What Is The Relationship Between Gas Pressure And Volume.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica What Is The Relationship Between Gas Pressure And Volume As the pressure on a gas increases, the volume of the gas. When it occupies a larger volume, it exerts a lower pressure. learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize. Use boyle's law to calculate. when a gas occupies a smaller volume, it exerts a higher pressure;. What Is The Relationship Between Gas Pressure And Volume.
From schoolworkhelper.net
Lab Answers Relationship Between Pressure and Volume of a Gas What Is The Relationship Between Gas Pressure And Volume the relationship between pressure and volume: define the relationship between gas volume and pressure, boyle's law. Use boyle's law to calculate. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. When it occupies a larger volume, it exerts a lower pressure (assuming the amount of.. What Is The Relationship Between Gas Pressure And Volume.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket What Is The Relationship Between Gas Pressure And Volume the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. the relationship between pressure and volume: define the relationship between gas volume and pressure, boyle's law. Use boyle's law to calculate. As the pressure on a gas increases, the volume of the gas. when a. What Is The Relationship Between Gas Pressure And Volume.
From www.grc.nasa.gov
Specific Volume What Is The Relationship Between Gas Pressure And Volume the relationship between pressure and volume: When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. when a gas occupies a smaller volume, it exerts a higher pressure; As the pressure on a gas increases, the volume of the gas. when a gas occupies a smaller volume, it exerts a higher pressure;. What Is The Relationship Between Gas Pressure And Volume.
From www.vecteezy.com
Boyle's Law, Relationship between pressure and volume of gas at What Is The Relationship Between Gas Pressure And Volume when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure. As the pressure on a gas increases, the volume of the gas. when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure. What Is The Relationship Between Gas Pressure And Volume.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector What Is The Relationship Between Gas Pressure And Volume when a gas occupies a smaller volume, it exerts a higher pressure; the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. define the relationship between gas volume and pressure, boyle's law. Use boyle's law to calculate. learn about and revise particle motion, gas pressure. What Is The Relationship Between Gas Pressure And Volume.
From www.youtube.com
Gas Pressure and Volume GCSE Physics Revision YouTube What Is The Relationship Between Gas Pressure And Volume when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure. When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. As the pressure on a gas increases, the volume of the gas. the volume (v) of an ideal gas varies. What Is The Relationship Between Gas Pressure And Volume.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas What Is The Relationship Between Gas Pressure And Volume When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. define the relationship between gas volume and pressure, boyle's law. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. When it occupies a larger volume, it exerts a lower pressure. . What Is The Relationship Between Gas Pressure And Volume.
From www.alamy.com
Boyle's Law , Relationship between pressure and gas volume Stock Vector What Is The Relationship Between Gas Pressure And Volume the relationship between pressure and volume: when a gas occupies a smaller volume, it exerts a higher pressure; When it occupies a larger volume, it exerts a lower pressure. Use boyle's law to calculate. the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number. As the. What Is The Relationship Between Gas Pressure And Volume.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube What Is The Relationship Between Gas Pressure And Volume define the relationship between gas volume and pressure, boyle's law. When it occupies a larger volume, it exerts a lower pressure. the relationship between pressure and volume: When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. As the pressure on a gas increases, the volume of the gas. when a gas. What Is The Relationship Between Gas Pressure And Volume.
From www.shalom-education.com
Volume and Pressure in Gases GCSE Physics Revision What Is The Relationship Between Gas Pressure And Volume When it occupies a larger volume, it exerts a lower pressure (assuming the amount of. when a gas occupies a smaller volume, it exerts a higher pressure; As the pressure on a gas increases, the volume of the gas. Use boyle's law to calculate. define the relationship between gas volume and pressure, boyle's law. learn about and. What Is The Relationship Between Gas Pressure And Volume.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint What Is The Relationship Between Gas Pressure And Volume When it occupies a larger volume, it exerts a lower pressure. when a gas occupies a smaller volume, it exerts a higher pressure; learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize. Use boyle's law to calculate. define the relationship between gas volume and pressure, boyle's law. . What Is The Relationship Between Gas Pressure And Volume.